Supplements answered: how to address those common customer questions.
Feed supplements are an important part of any successful feed business, but can we always answer those customer questions? ETN asked Kate Hore RNutr (Animal), Head Nutritionist at NAF, to help.

AMTRA is required by the Veterinary Medicines Regulations to ensure its RAMAs/SQPs undertake CPD. All RAMAs/SQPs must earn a certain number of CPD points in a given period of time in order to retain their qualification. RAMAs/ SQPs who read this feature and submit correct answers to the questions below will receive two CPD points. For more about AMTRA and becoming a RAMA/SQP, visit www.amtra.org.uk
Q: How can we trust the label – are there legal controls on these products?
KH: It is a common misconception that supplements are somewhat unregulated, and manufacturers can do as they like. However, this is simply not true. Legally there is no such thing as a ‘supplement’; they all come under Feed Law. Therefore, it doesn’t matter whether we’re looking at a powder, liquid, syringes or treats they are all viewed simply as feed, and come under the same suite of regulations that bagged compound feeds have to adhere to. Anyone producing supplements must be a registered feed manufacturer, and adhere to retained EC Regulation 767/2009 on production, labelling, legal marketing and claims.
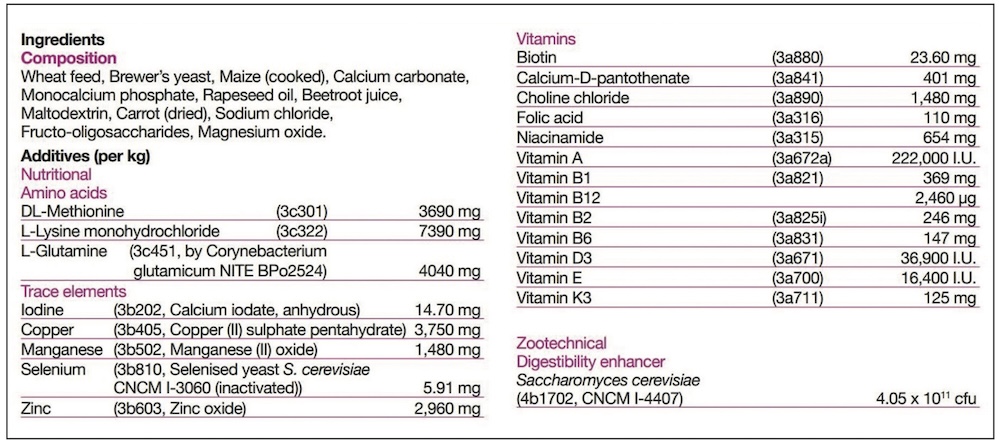
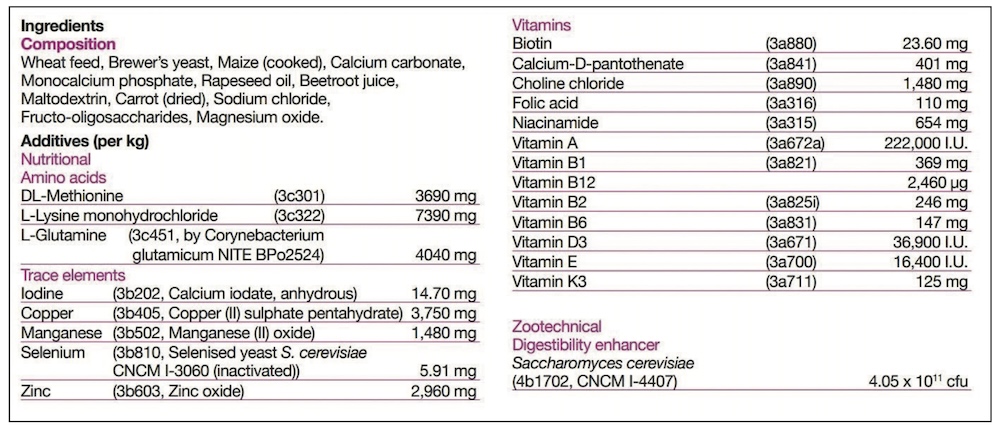
Generally, feed supplements will fall into one of the following feed definitions, and the appropriate one must be declared on the label.
- Complementary Feed: A blend of two or more feed materials, which contributes to the daily ration when used in combination with other feeds.
- Feed Material: A single ingredient in its natural state, intended to meet nutritional needs.
- Mineral Feed: A complementary feed high in minerals, which is seen by having an Analysis over 40% crude ash.
Q: Why do they contain Additives? Some of my customers are wary of them.
KH: Under the Ingredients section of the label there will be two basic areas. The ‘Composition’ is the listing of the ‘Feed Materials’, those ingredients designed to meet the nutritional needs. Typically for feed supplements these would include the herbs, oils, major minerals such as calcium or magnesium sources, yeasts, joint support ingredients and cereals. Separately the ‘Additives’ are listed, and these are simply technical ingredients. Depending on the formulation, the product may, or may not, require additives – so some will have none listed. Additives are nothing to be concerned about, and may include vitamins and trace elements to balance the diet, probiotics or quality additives like preservatives and flavours.
Just as with our own food, it is important to ensure that the product, once packaged, remains in good condition, safe and effective for your customer’s horses for the length of its shelf life. These technical ingredients can be invaluable in maintaining quality, or simply ensuring the product can do what we want it to do.
Q: How can we tell which are the key ingredients in a supplement, or how much is included?
KH: It slightly depends on what is meant by a ‘key ingredient’, and whether it is a Feed Material (in Composition) or Additive. For Additives, it is simple, as their inclusion rate is always listed alongside. The Additives will also have numbers listed, and these are simply their relevant registration numbers.
An additive might be a key ingredient without being included at a high rate. For example, selenium is essential to general health, and particularly muscle health, but is only required at very small amounts. Therefore selenium may be marketed as a key ingredient, but is never going to be a large ingredient. However, its additive listing will clearly tell you how much is included.
For feed materials it is not quite so clear, but there are still legal guidelines. Legally there is no requirement to list the inclusion rates of all ingredients under the Composition section; and this is right and fair as to do so would, effectively, give away the manufacturer’s unique formulation. However there are two things you can look out for to help you advise your customer.
Firstly, whilst we don’t have to declare the levels, but there is a legal requirement to list all feed materials in inclusion order from the largest down to the smallest. Take a look, and if the first ingredient is ‘Dextrose’, for example, it is principally a pot of sugar!
Secondly, if an ingredient is highlighted in marketing, for example ‘With added dried rosehips’, then you are legally required to declare the level of that ingredient. The level may be included with the ‘Composition’ listing, or may be detailed elsewhere on the label, but the level of that highlighted ingredient must be declared.
Q: If a customer only wants to feed plant-based ingredients, is there anything I should look out for?
KH: This is an interesting one, as feed materials for horses may be of animal origin, including traditional supplements such as Cod Liver Oil from fish. Joint support is an area that particularly tends to use ingredients of animal origin. Whilst this can be perfectly safe and suitable, some owners will want to avoid those ingredients, either as vegetarians themselves or they understand horses to be, and so what are their choices?
Glucosamine
There are several types of glucosamine from different sources. For glucosamine the legislation helps you advise the customer as, legally, the label must list the source. It is particularly important here as glucosamine can be either animal origin, from crustaceans or other arthropods, or vegan from fermentation of grain. Therefore, under the ‘Composition’ heading you would expect to see glucosamine listed with its source. If that source is not declared you should contact the manufacturer to ask them why it is not, as it is a legal requirement.
Chondroitin sulphate and Collagen
For these ingredients there is some consistency as – by definition – both chondroitin and collagen are always of animal origin. For collagen, like glucosamine, there is a legal requirement to include the source of that collagen on label, often from birds. However, somewhat unusually, the same does not apply to Chondroitin sulphate. Therefore, you may not see the source declared, but it will be of animal origin, typically avian or porcine.
Q: This supplement is 16% Crude Protein – is it too high for a sensitive horse or pony?
KH: This is a relatively common question, particularly as protein may be elevated due to yeasts or concentrated amino acids, for example. However, where supplements are concerned, protein levels are almost never a cause for concern, as percentages alone mean nothing without also considering
the feeding rate. It is a legal requirement to list protein levels on feed, which is why it should be on supplement labels. But with the concentrated feeding rates of supplements, typically only 10-100g per day, in a 10kg diet (500kg horse), the actual protein level is meaningless.
For example, a 16% protein supplement fed at 24g daily provides just 3.8g crude protein.
Compare that to the protein requirements of the horse, i.e. a 500kg horse in moderate work requires 768g protein per day*, you can see levels in the supplement do not impact significantly.
Conclusion In short, feed supplements are simply regulated feeds specialised with highly concentrated feeding. We hope to have answered some of the most common questions here, but do contact NAF for any further information.

ABOUT ETN’s RAMA/SQP FEATURES
ETN’s series of CPD features helps RAMAs (Registered Animal Medicines Advisors/SQPs) earn the CPD (continuing professional development) points they need. The features are accredited by AMTRA, and highlight some of the most important subject areas for RAMAs/ SQPs specialising in equine and companion animal medicine.
AMTRA is required by the Veterinary Medicines Regulations to ensure its RAMAs/SQPs undertake CPD. All RAMAs/SQPs must earn a certain number of CPD points in a given period of time in order to retain their qualification. RAMAs/ SQPs who read this feature and submit correct answers to the questions below will receive two CPD points. For more about AMTRA and becoming a RAMA/SQP, visit www.amtra.org.uk











